
In the context of global warming, the "low-carbon economy" based on low energy consumption and low pollution has become a global hot spot. Developed countries in Europe and the United States have vigorously promoted the "low-carbon revolution" with high energy efficiency and low emissions as the core, focused on the development of "low-carbon technologies", and made major adjustments to industrial, energy, technology, trade and other policies in order to seize the opportunities and industrial commanding heights. The battle for a low-carbon economy has quietly started around the world.
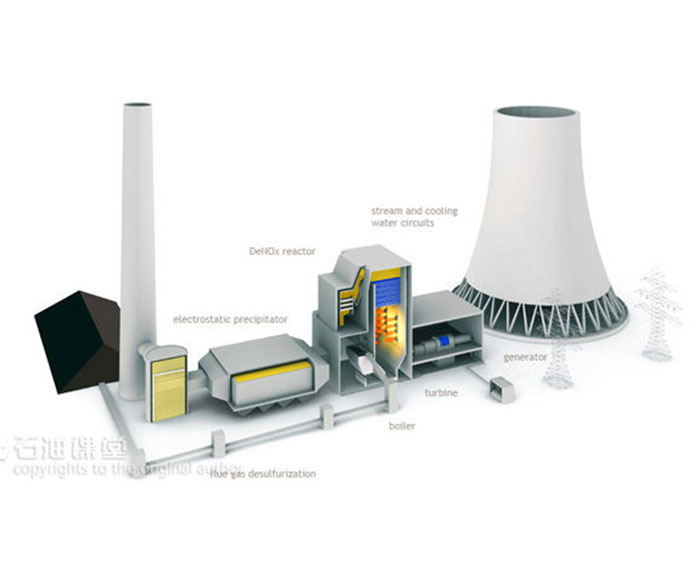
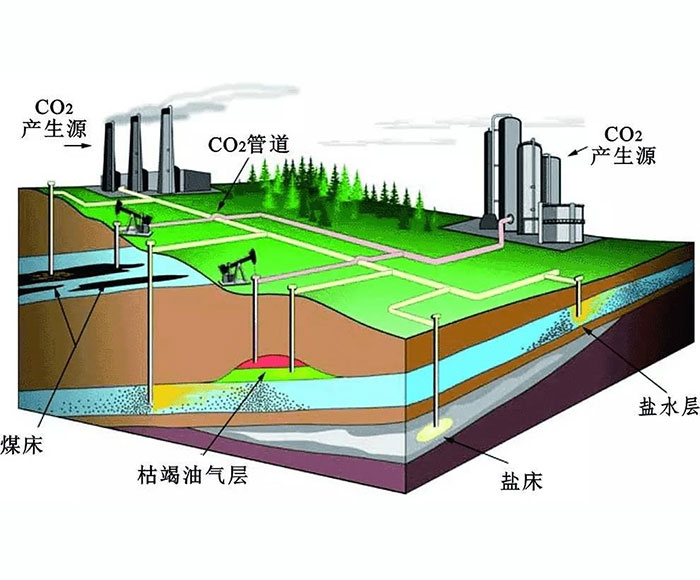
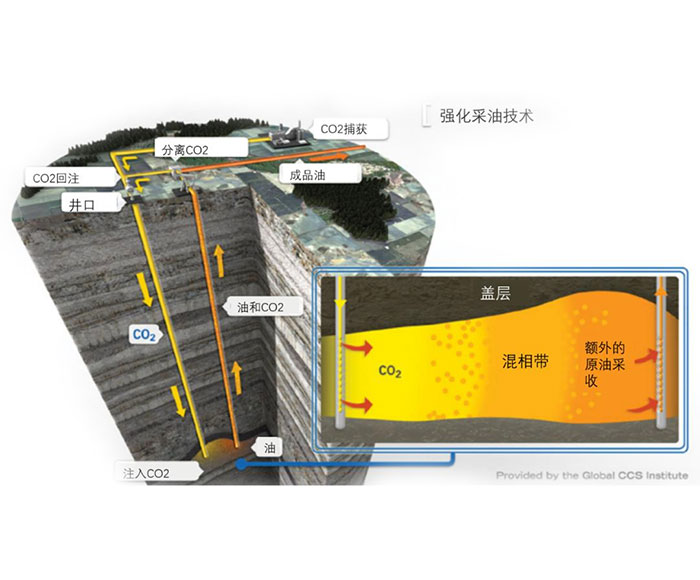
Amine absorption method: The essence of amine absorption method is acid-base neutralization reaction, the reaction of weak acid (carbon dioxide) and weak base (amine in aqueous solution) to produce water-soluble salt, which makes the amine method can capture CO2 in the flue gas at a lower partial pressure level, but the absorption load is limited by the reaction balance. The acid-base reaction is reversible with temperature, generally forming salts at about 40 ° C, and CO2 is absorbed. When the reaction is above 100℃, the CO2 is released in reverse, so the CO2 can be captured by using the absorption tower and the component system in the Santa. However, due to the high content of water in the circulating absorption solution (>50%), its periodic heating and cooling make the system energy consumption too high.
The alcoholamines used in the amine process include traditional chain substituents and branched steric hindrance amines. According to the number of N atoms, alcohol amines are divided into primary amines (such as ethanolamine, MEA), secondary amines (such as diethanolamine, DEA), tertiary amines (such as triethanolamine, TEA); Diisopropanolamine (MDEA), each of which has advantages in the absorption of CO2. For example, MEA has small molecular weight, fast absorption rate of CO2, and the most advantageous for capturing low concentration CO2 in flue gas, so it is also the most studied and used, but limited by reaction balance, its absorption load is the smallest. In addition, in order to control the oxidative degradation of amines and their corresponding corrosion problems, it is also necessary to add certain antioxidants and corrosion inhibitors to the absorption solution, and there are certain requirements for the flue gas (generally required to have completed deoxidation, dust removal and desulfurization). Although the amine absorption process has been used in the chemical industry for many years, especially to separate acidic gases from natural gas, there is still room for technological advancement: Current research focuses on the development of efficient (mixed) absorbers and their additives, the development of efficient new tower internals for large separation equipment, the optimization of process parameters and thermal integration in power plants, and the improvement or innovation of regeneration processes, focusing on solving the problems of high energy consumption and high cost in the capture process.
The process system of the ammonia water method is similar to that of the amine absorption method. The key reaction step is ammonium carbonate, carbon dioxide and water to produce ammonium bicarbonate. Compared with amine system, this reaction has a lower reaction heat, so it has the advantage of low renewable energy consumption. In addition, compared with amine method, it also has the advantages of fast absorption rate, strong absorption capacity, anti-oxidation degradation, low corrosion and cheap price. However, due to the high volatility of ammonia, in the absorption stage, the flue gas needs to be cooled to 15-26℃ to improve the absorption of CO2 by ammonia and avoid ammonia escape. In the regeneration stage, due to the need for temperature rise, the control of ammonia escape is more urgent, which is a problem that the current ammonia method needs to focus on.
Hot alkali method, another promising absorption/regeneration method is the hot potassium alkali method with piperazine as the activator. Because the operating temperature of the absorption/regeneration process of this method is not much different, the regeneration heat consumption is greatly reduced compared with the conventional amine method.
The absorption membrane method is generally a new absorption process that combines the microporous membrane (mostly hollow fiber membrane) with the amine absorption method. Gas and absorption liquid flow on both sides of the membrane without direct contact, the membrane itself is not selective, only plays the role of isolating gas and absorption liquid: The pores on the microporous membrane are large enough to allow the gas molecules separated on one side of the membrane to pass through the membrane to the other side without high pressure, which mainly relies on the selective absorption of the absorption liquid on the other side of the membrane to separate a certain component of CO2 in the gas mixture. The advantages of this method in mass transfer performance, operation and energy consumption make it one of the hot spots in research and development.
Chemical adsorption method, this method uses the selective reversible adsorption of CO2 in the mixture of solid adsorbents to separate and recover CO2, such as alkaline metal oxides can absorb acidic CO2 gas to produce carbonate, and the reaction can be reversed at higher temperatures. Currently, more research is on lithium compound adsorbents. A solid adsorbent can also be made by loading an active substance that reacts easily with CO2 onto a porous solid (also known as "absorbent curing"). Due to the high specific surface area of the carrier, sufficient contact area is provided for the contact reaction between the active substance and CO2, and the process does not contain liquid water compared to the amine solution absorption method, thus having the potential for low energy consumption.
Ionic liquid is a new type of "green solvent" composed of specific cations and anions, which is liquid at or near room temperature. Numerous studies have shown that ionic liquid has stable properties, non-volatilization, strong CO2 absorption capacity, easy separation of products, high recyclability and designability of cations and ions, etc., and is considered as a substitute for volatile organic compounds. In recent years, CO2 recycling has attracted much attention. The ionic liquid has almost no vapor pressure, which can effectively alleviate the pollution problem caused by VOCs emission in the organic amine absorption method. At the same time, because of its good thermal stability, the ionic liquid can be analyzed and recycled at a lower temperature. More importantly, it can target CO2 gas. The functional ionic liquid with high initial absorption capacity was designed and synthesized according to the practical requirements.